Elaboration of Nanoparticles (NPs)
I am developing nanoparticles for various applications with a focus on in depth characterizations for control of physicochemical properties with robust, reproducible and up-scalable methods.
Biological interactions of NPs
I am studying biological interactions of engineered nanoparticles especially in medical imaging and nanotoxicology. I am focusing on innovative bio-analysis methods for nanohybrids.
Proteins / NPs interactions
I am focusing part of my research on the fascinating topic of interaction between nanoparticles and proteins « the protein corona » with the ultimate goal to control it to improve nanomedical solutions
Elaboration of Nanoparticles
Functionalized Nanoparticles

During different research and industrial projects, diverse nanoparticles (NPs) are synthesized with robuste and reproducible protocols. These NPs are also surface functionalized to improve their biocompatibility and their specificity for the applications targeted.
Chemical approaches to modify NP’s surfaces are optimized depending on the expectations with always an important part dedicated on developing biocompatible and safe methods.
Various NPs are shortly presented below.
Superparamagnetic iron oxide NPs: SPIONs
SPIONs are used for their magnetic properties for example in medical imaging (MRI) or for separation

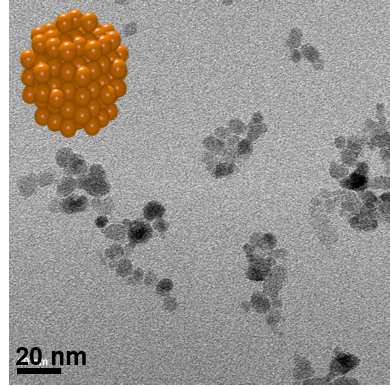
Gold NPs (AuNPs) with modulating sizes
Gold NPs can be used in medical imaging or therapy: theragnostics

Polymeric nano-bubbles known as polymersomes
Polymersomes can trapped drug and release it at will in the case of in vitro therapeutical approaches
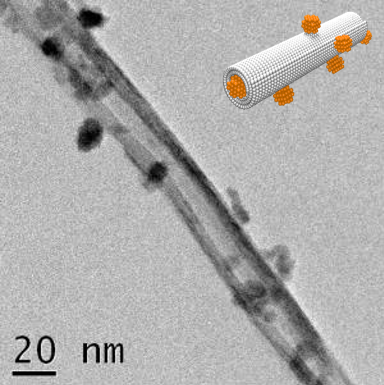
Titanate nanotubes (TiONTs) with SPIONs
TiONTs demonstrated efficacy in DNA transfection of radiotherapy
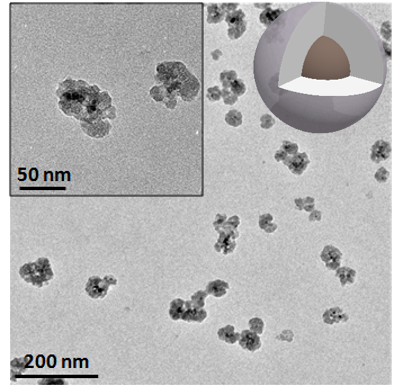
Core/shell structure of silica encapsulating SPIONs
Silica/SPIONs combined drug release of silica shell and magnetic properties of the SPIONs core

Carbon encapsulating Iron NPs (Fe@C)
Fe@C can be also used in medical imaging and hyperthermia
In depth Characterizations
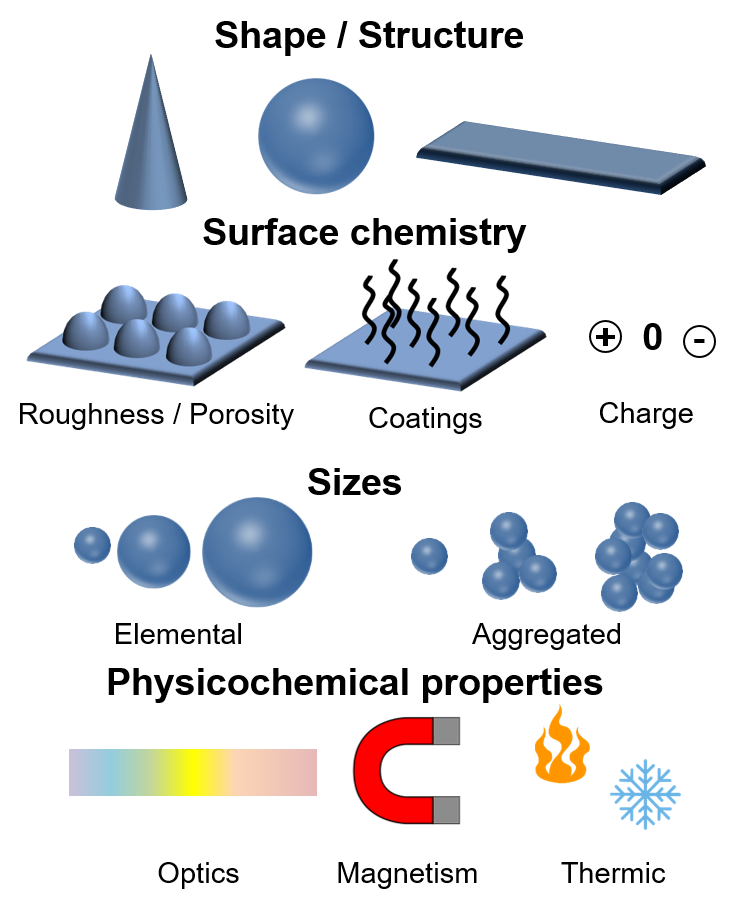
To prevent any variability during the use of nanoparticles or nanomaterials, characterization is a crucial step. My skills are to be able to analyze the material at the nano- and the macro-scale.
Shape is an important parameter as well as crystallographic structures.
Interfaces between the material and its environnement generates reactions. Thus, roughness, porosity and charge has to be analyzed. Quantity of surface functionalizations has to be rigorously quantified.
Sizes are also important for NPs. We could distinguish elemental size and hydrodynamic size.
Physicochemical properties must also be characterized.

X-Ray Diffraction (XRD)
XRD allows to determine crystallographic structures and the elemental size of nanoparticles

TGA
Thermogravimetric analyses measure the amount of organic molecules on a surface to be able to quantify the coatings

Electronic Microscopy (TEM)
TEM gives images to estimate size and shapes of nanomaterials and can also do elemental analyses
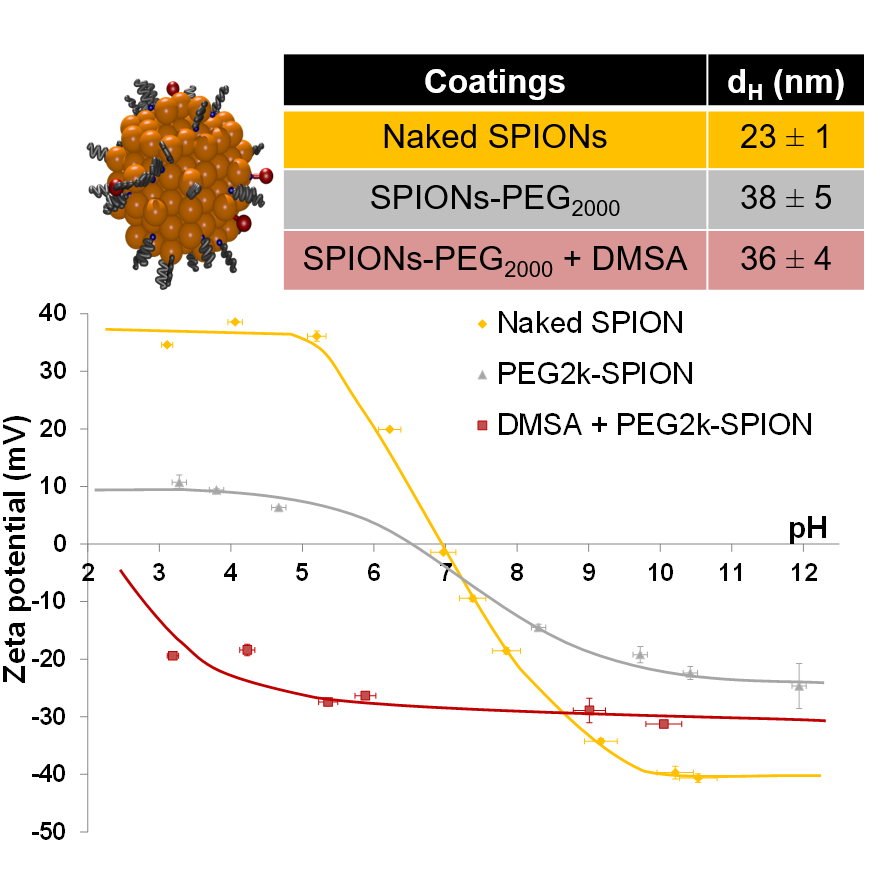
DLS & Zeta potential
Dynamic Light Scattering gives sizes of NPs in liquid a size closer to what « environment sees » than elemental size.
Zeta gives surface charge
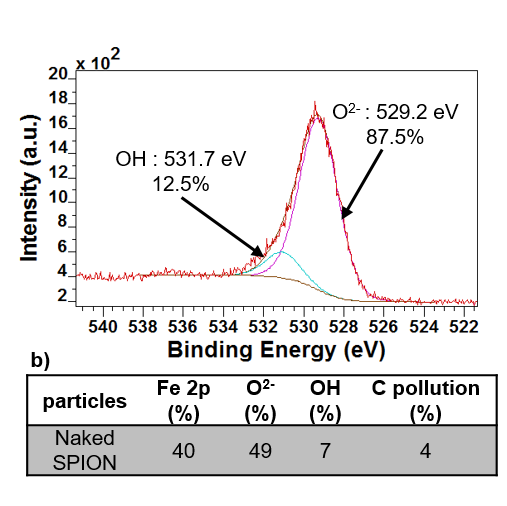
XPS
X-Ray Photoelectron Spectroscopy analyzes material’s surface such as the quantity of organic coatings

Magnetic Hysteresis
Magnetic hysteresis measurements can quantify if the SPIONs, for example, are superparamagnetic
Industrial Approaches
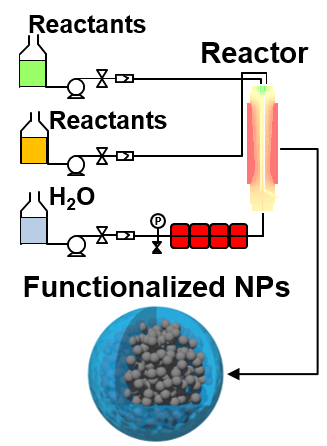
For the applications targeted and during national or international projects, it is crucial to control synthesis steps and characteristics of the elaborated NPs.
Thus, I dedicated part of my research focusing on the development of robust and pre-industrial methods to obtain reproducible and reliable functionalized NPs.
Scale-up approaches via lab-made hydrothermal process as well as standard operating procedures are developed in addition to the systematic in depth characterizations presented above.
Some pre-industrial NPs are presenting below. For the biomedical project, all these NPs are composed of iron oxide.
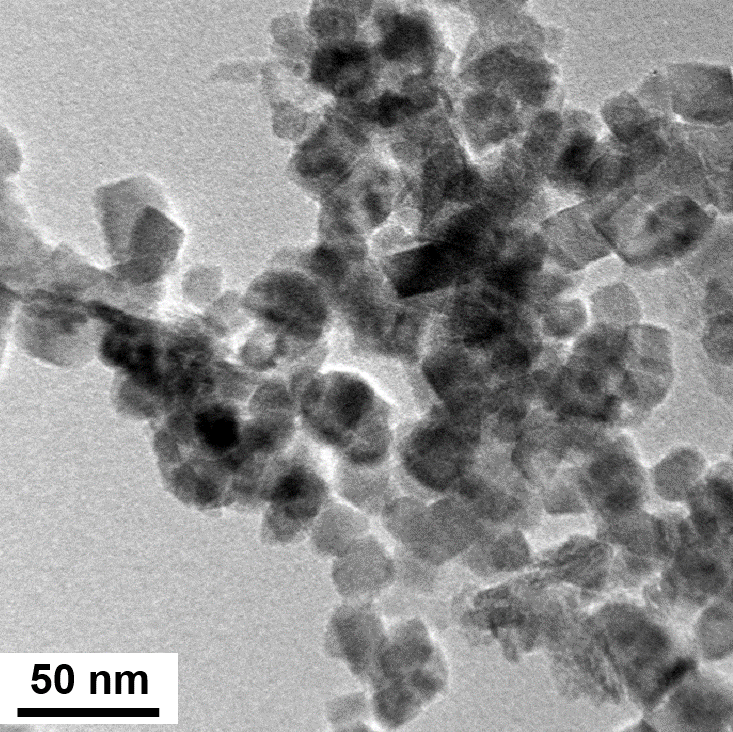
Iron oxide NPs with elemental size at ~20 nm ideal for hyperthermia applications.

Silica/iron oxide core/shell NPs obtained in large quantity and reproducibly.

Iron oxide NPs obtained in supercritical water in a reaction time below 10 seconds.
Biological interactions of NPs
NPs for medical diagnosis

The nanoparticles developed during many research ad industrial project are mainly composed of iron oxide (SPIONs) with original magnetic properties.
These SPIONs were used as medical imaging contrast agents in MRI (Magnetic Resonance Imaging) with medical partners in France and Europe and in MPI (Magnetic Particles Imager) during my visit at Stanford medicine in 2022.
The SPIONs elaborated were used directly in injection to target liver in MRI and MRI coupled to PET-scan instruments. SPIONs also labeled cells to follow diagnosis or therapeutic effects in MRI.
Some significant studies are given below.
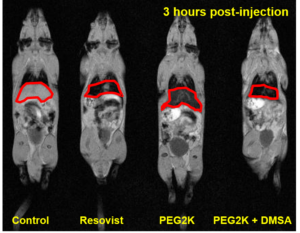
MRI contrast agents
SPIONs with different surface coatings have different liver uptake.

Stem cells engraftment
Labeling stem cells with SPIONs allows following their engraftment by MRI.

Bimodal contrast agents
SPIONs functionalized with radioactive element to be detected in both MRI and PET-scan simultaneously: a clinical instrument at CHU Dijon.
Nanotoxicity
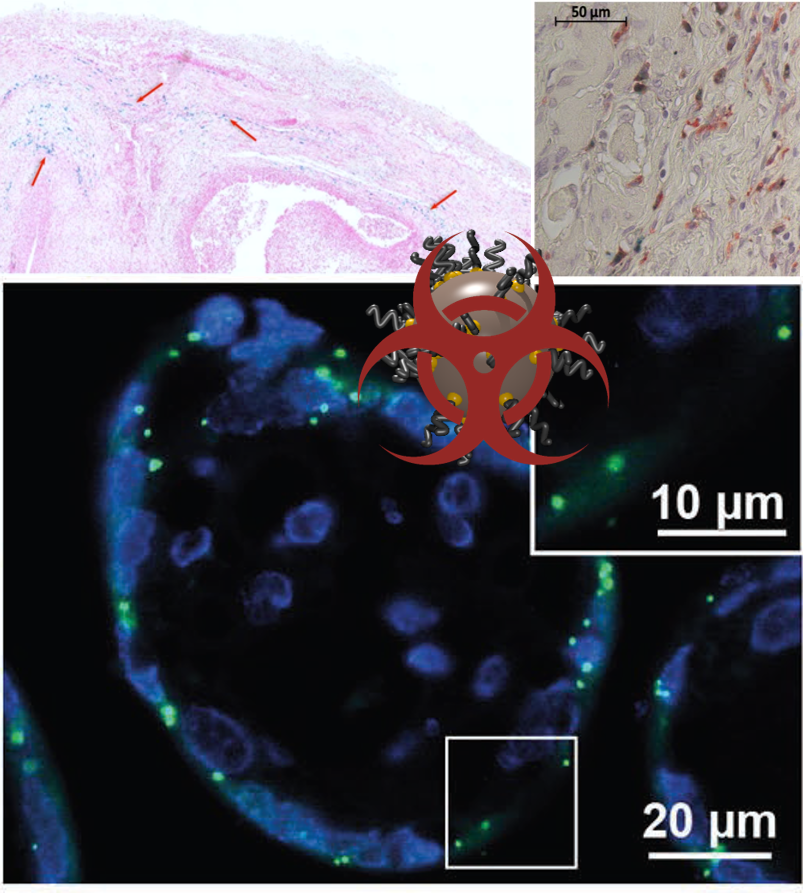
An important question when working with NPs is: are they safe ?
This simple question rises many scientific studies to correlate the surface properties of the NPs. IN fact, it is at the interface of NP’s surface and bio-environment that biological reactions starts.
It is then crucial to study the role of NPs’ functionalizations in cellular reactions such as toxicity, oxidative stress or uptake as well as in vivo toxicity and biodistribution.
Standardization of nanotoxicology is also an important parameter to focus on as their is, for the moment, no standard procedure in this discipline.
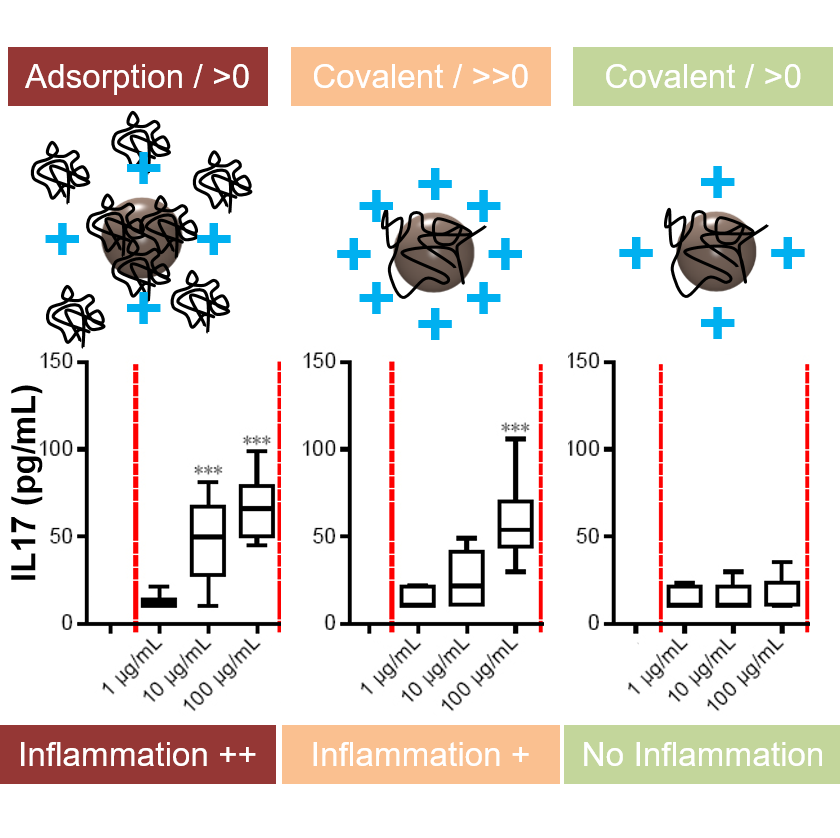
NPs’ surface vs nanotoxicology
Surface coatings influence inflammatory reaction. Covalent coatings are less toxic than adsorption. Highly positive charge induces more toxicity.

NP’s surface vs Cell uptake
Depending on the polymer chosen to coat NPs, cellular uptake in immune and hepatic cells are different.

Standards for nanotoxicology
NPs interact with classical biological tests.
Finding NPs (Zinc oxide for example) as negative and positive control is crucial.
Proteins / NPs interactions: The Protein Corona (PC)
Physicochemistry influences PC

Studying biological interactions of NPs in a classical biological way is not sufficient. In fact, once incubated in any biological fluids NPs will instantly react with biomolecules. Proteins are the most important component of biofluids and will cover NP’s surface forming a protein corona (PC).
I am studying this quite recent topic (< 15 years) because I do believe it is crucial to understand proteins / NPs interactions in order to control biological behaviors of NPs.
Firstly, with my nanomaterials skills, I focus on the role of the surface chemistry on protein’s adsorption.
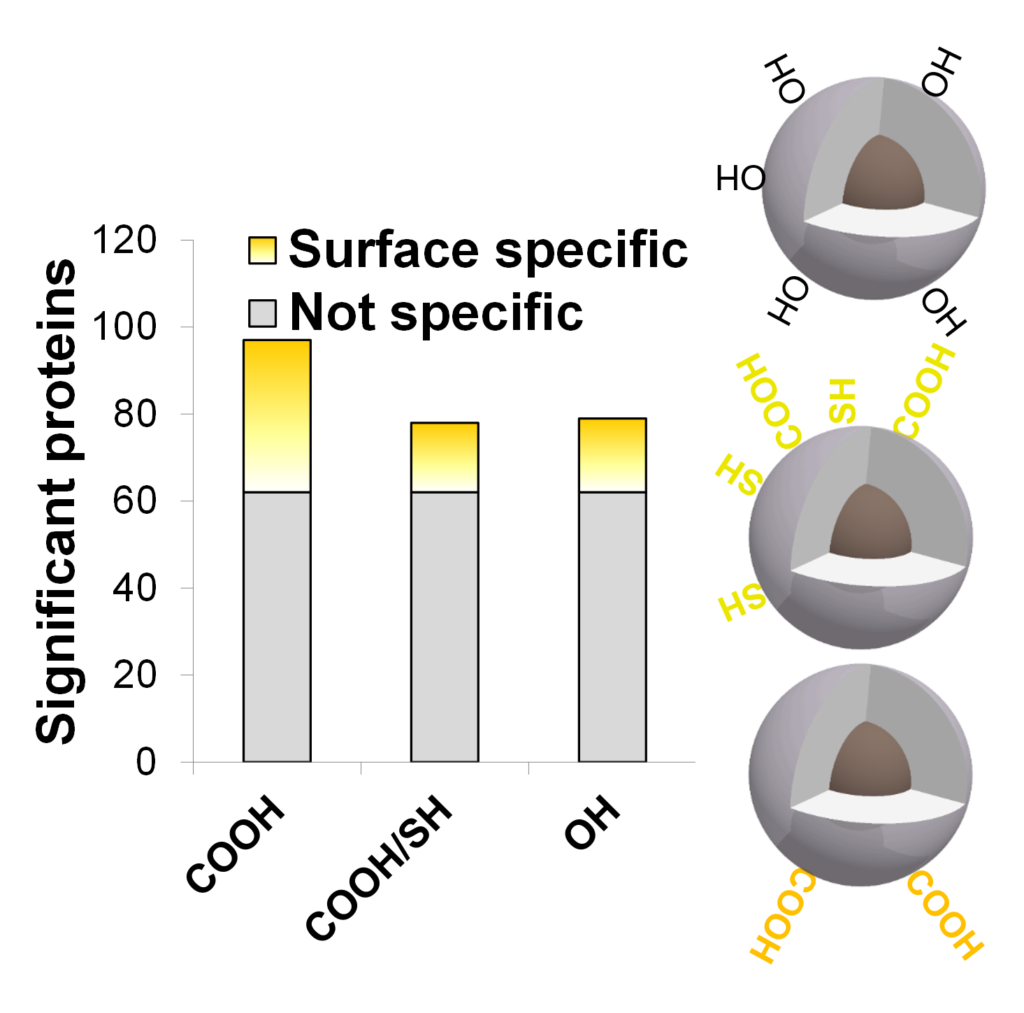
Role of charge on NPs protein corona
Proteins found on NP’s surface have more interactions with their charge (not specific) than chemistry (surface specific).

Various chemistries / various abundant proteins
Focusing on the 5 most abundant proteins present in serum, the chemistry influenced the number of proteins found on NP’s surface.
Biological role of PC
What is fascinating about studying the protein corona (PC) formed onto NPs is that it is influenced by NP’s surface but it also influences NP’s biological behaviors.
In this axis of my research, I am focusing on understanding the role of the PC on NPs in vivo behaviors. Despite this subject it is quite confidential with less than 20 publications worldwide.
In the following examples, I will describe the most interesting and recent progress we made.

In vivo & in vitro PC are not reliable
PC formed in vivo is really different than thus formed in vitro leading to misinterpretation of many in vitro studies on protein corona.
Focusing on proteins / NPs interactions in vivo will accelerate technology transfert of nanomedicine.
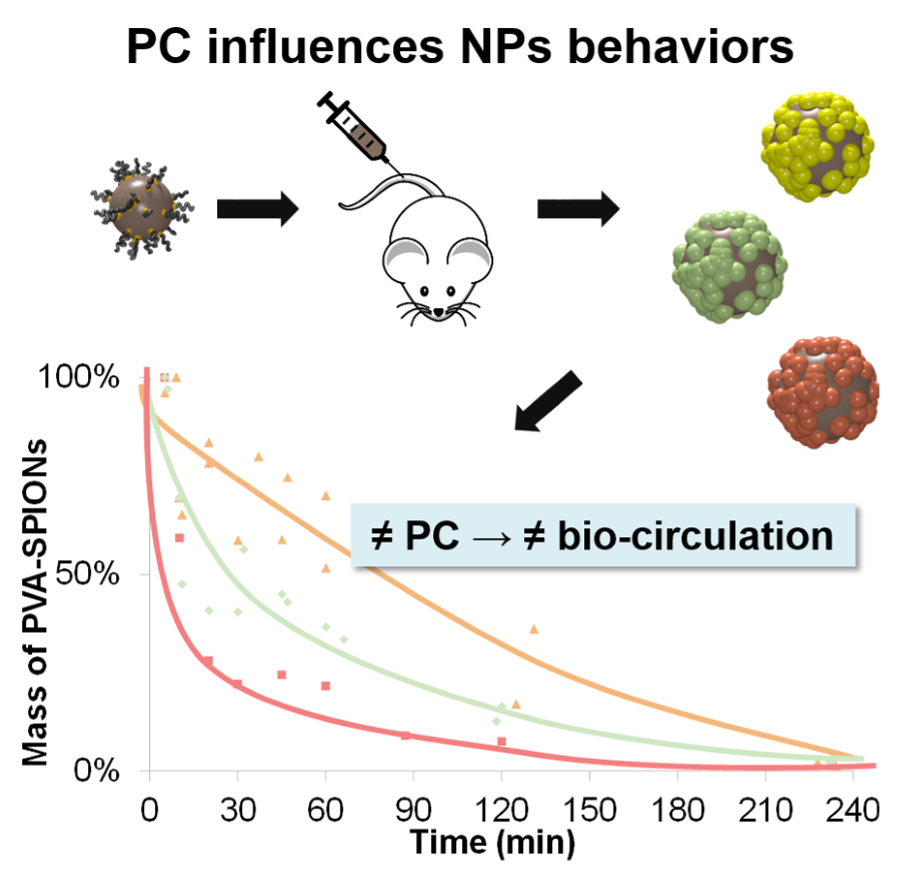
PC influences in vivo behaviors of NPs
Proteins found in vivo on the surface on NPs influence their bio-circulation. PC is linked to the surface chemistry of the NPs (polymers, charges…).
Standardization of PC

Studying protein corona (PC) is a challenging and intriguing topic. As it is a recent area of research there are still many possible discoveries with potential strong impact of the future of nanomedicine.
As biological fluids, especially the blood, are complex fluids, It is however crucial to standardize proteins separation and analyses to avoid biased results leading to misunderstandings.
With my nanoscientist background, when studying PC, I am also focusing on developing standardized and robust methods to trust the final results.
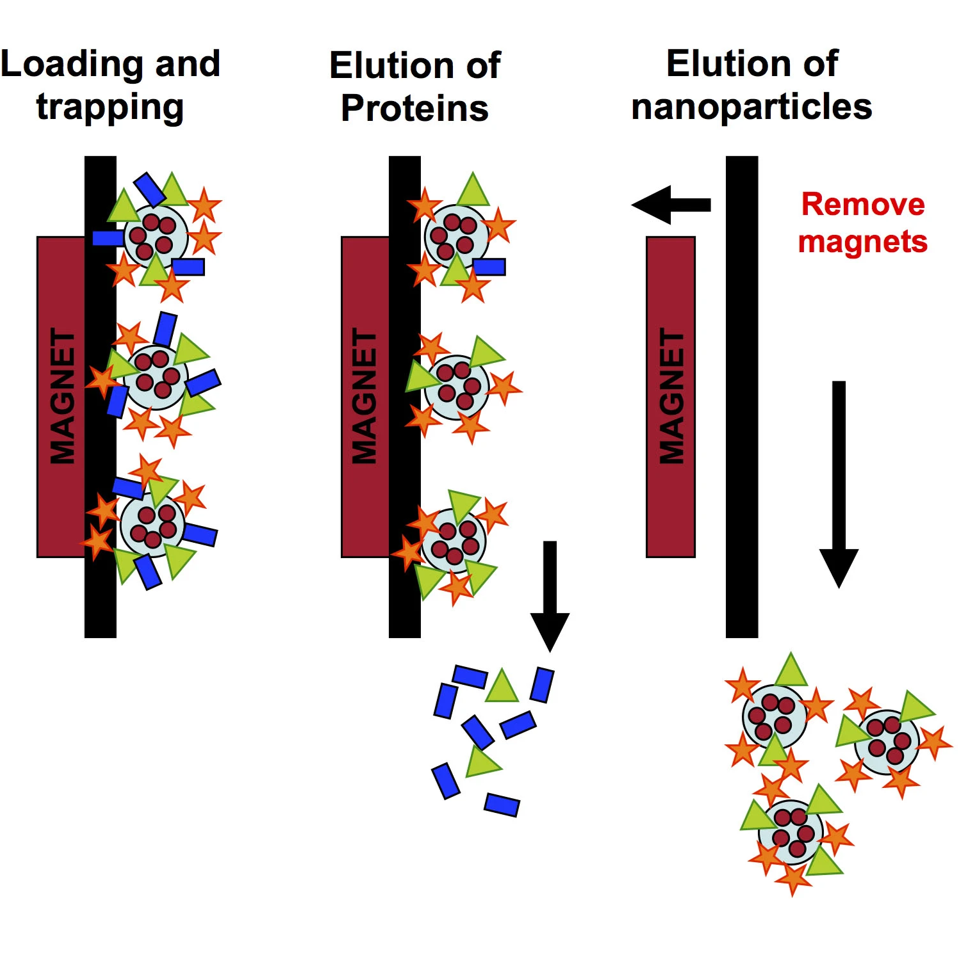
Optimized proteins separation device
A magnetic separator was developed to trap magnetic NPs once incubated in biological fluids and then remove the not strongly adsorbed proteins to be able to analyze the so-called « hard protein corona ».

Control and replicates
As it is always the case in scientific studies, it is important to properly run control experiments and replicates. This obvious observation is not always done in PC studies leading to biased observations and conclusions.